Chapter 10 - Petrological thermodynamics of liquid and solid phases¶
%matplotlib inline
import matplotlib.pyplot as plt
import matplotlib.tri as mtri
import numpy as np
import numpy.matlib
from scipy.optimize import fsolve
from scipy.interpolate import interp1d
import warnings
warnings.filterwarnings('ignore')
The equilibrium state¶
The partition coefficient \(\equi{\parcod}_j\) is given by
In cases where, for example, we use a small set of fictitious effective components to approximate the full thermochemical system, the error that we make in taking \(\phasemolarmass\liq/\phasemolarmass\sol \approx 1\) is acceptably small. With this approach, the partition coefficient \(\equi{\parcod}_j\) becomes
where \(\latent_j\equiv-\Delta\enthalpy_j^m\) is the latent heat of melting for a solid composed of pure component \(j\) and \(R_j \equiv R/M_j\) is the modified gas constant.
Partition coefficients \(\parco_j\) give an implicit equation for the equilibrium melt fraction \(\equi\mf\) as
Once a value of \(\equi\mf\) has been numerically obtained, it can be used to determine the phase composition
Table below shows parameter values for the two- and three-component ideal-solution systems. These values are useful for the demonstration of ideal-solution phase diagrams but should not be taken as an optimal calibration for the mantle.
Name |
\(j\) |
\(\melttemp_j(P=0)\) [\(^\circ\)C] |
\(\clapeyron_j\) [GPa/K] |
\(\latent_j\) [kJ/kg] |
\(R_j\) [J/kg/K] |
|---|---|---|---|---|---|
olivine |
1 |
1780 |
1/50 |
600 |
70 |
basalt |
2 |
950 |
1/100 |
450 |
30 |
hydrous basalt |
3 |
410 |
1/50 |
330 |
30 |
Application to a two-pseudo-component system¶
The change of this melting temperature with pressure is given by the Clausius-Clapeyron equation. For simplicity, we assume a constant value of \(\clapeyron = \Delta\entropy/\Delta(1/\density)\), which gives
This produces the straight lines in Figure below (a) under the assumption that the thermodynamic pressure mis lithostatic, i.e., \(\Grad\pres=\density\gravity\).
class IdealSolutionParameters:
def __init__(self, L, R, clap, Tm0):
self.L = L # J/kg
self.R = R # J/kg/K
self.clap = clap # GPa/K
self.Tm0 = Tm0 # K
class ThetaStructure:
def __init__(self, f=0, cl=None, cs=None):
self.Ts = 0.0
self.Tf = 0.0
self.f = f # f the melt fraction
self.cl = cl # liquid concentration vector (length n)
self.cs = cs # solid concentration vector (length n)
def ParCoef_IdealSolution(T, P, par):
Tm = par.Tm0 + P/par.clap
return np.exp(par.L / par.R * (1./T - 1./Tm))
def EquilibriumResidual(TT, ff, PP, cc, par, parco_func):
K = np.array(
[parco_func(TT, PP, par_j) for par_j in par]
).flatten()
R = np.array(
[cc_j * (1. - K_) / (ff + (1. - ff) * K_) for cc_j, K_ in zip(cc, K)]
)
return R.sum()
def EquilibriumState(n, cbar, T, P, par, parco_func):
if n == 1:
raise Exception('n must be greater than 1')
if len(par) < n:
if len(par) == 1:
par = [par] * n
else:
raise Exception('par array of structures must be length 1 or n')
if isinstance(T, np.ndarray) and isinstance(P, float):
P = P * np.ones_like(T)
if isinstance(P, np.ndarray) and isinstance(T, float):
T = T * np.ones_like(P)
if isinstance(T, float) and isinstance(P, float):
r = cbar.shape[0]
T = np.ones((r,)) * T
P = np.ones((r,)) * P
if len(T) != len(P):
raise Exception(
f'length(T)={len(T)} and length(P)={len(P)}'
' must be equal if both are greater than 1'
)
r = len(T)
if cbar.shape != (r, n) and cbar.shape != (1, n) and cbar.shape != (n,):
raise Exception('one dimension of the cbar array must have length n')
if cbar.shape == (n, r):
cbar = cbar.transpose()
if cbar.shape == (1, r) or cbar.shape == (n,):
cbar = np.matlib.repmat(cbar, r, 1)
if cbar.shape != (r, n):
raise Exception('cbar must have shape ({}, {})'.format(r, n))
Theta = []
for T_i, P_i, cbar_i in zip(T, P, cbar):
Theta_i = ThetaStructure()
Theta_i.Ts = fsolve(
lambda T_: EquilibriumResidual(
T_, 0.0, P_i, cbar_i, par, ParCoef_IdealSolution
),
T_i
)[0]
Theta_i.Tl = fsolve(
lambda T_: EquilibriumResidual(
T_, 1.0, P_i, cbar_i, par, ParCoef_IdealSolution
),
0.5*(T_i+Theta_i.Ts)
)[0]
if T_i < Theta_i.Ts:
Theta_i.f = 0.0
elif Theta_i.Tl < T_i:
Theta_i.f = 1.0
else:
Theta_i.f = fsolve(
lambda f_: EquilibriumResidual(
T_i, f_, P_i, cbar_i, par, ParCoef_IdealSolution
),
0.0
)[0]
K = np.array([parco_func(T_i, P_i, par_j) for par_j in par])
Theta_i.cl = cbar_i / (Theta_i.f + (1. - Theta_i.f)*K)
Theta_i.cs = K * Theta_i.cl
Theta.append(Theta_i)
if len(Theta) != len(T):
raise Exception('inconsistent number of ThetaStructure')
return Theta
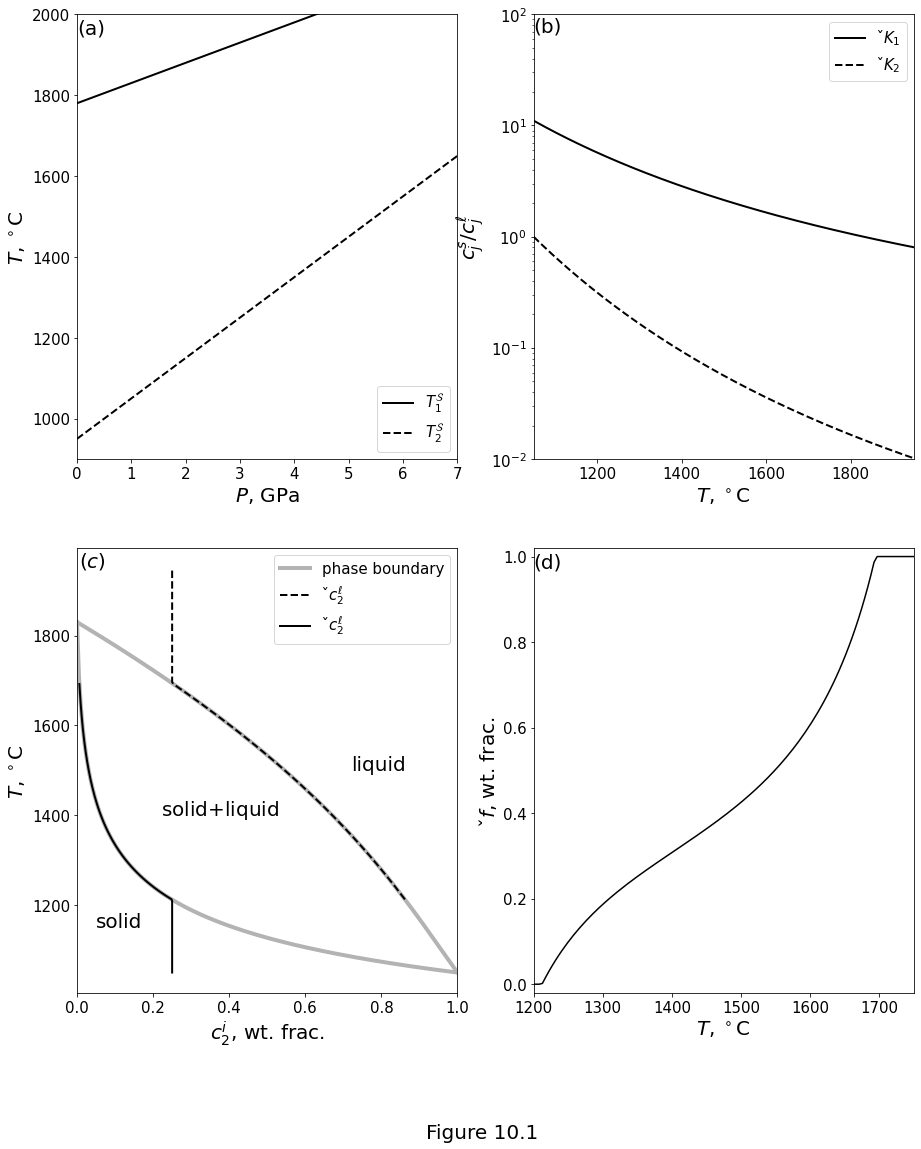
Figure 10.1 plots the ideal-solution model of a two-component system of olivine (\(j=1\)) and basalt (\(j=2\)). Parameter values are given in table above. (a) Pure-component melting temperatures \(\melttemp_j\) as a linear function of pressure. (b) Partition coefficients \(\parco_j\) as a function of temperature at a constant pressure of 1 GPa. (c) Grey lines are the solidus and liquidus temperature as a function of the basalt fraction at a pressure of 1 GPa. The composition along the solidus curve refers to the solid phase; that along the liquidus curve refers to the liquid phase. Black lines indicate the compositional evolution of a closed system with bulk composition of 25% basalt component. (d) Equilibrium melt fraction as a function of temperature for a bulk composition of 25% basalt component at 1 GPa.
par = [
IdealSolutionParameters(600e3, 70., 1./50., 1780. + 273.),
IdealSolutionParameters(450e3, 30., 1./100., 950. + 273.)
]
# thermodynamic state
Pref = 1.
Pvar = np.linspace(0., 7., 20)
Tref = 1350. + 273.
Tvar = np.linspace(1050., 1950., 200) + 273
cref = np.array([0.75, 0.25])
cvar = np.zeros((200, 2), dtype=float)
cvar[:, 0] = np.linspace(0., 1., 200)
cvar[:, 1] = 1.0 - np.linspace(0., 1., 200)
fig, ((axA, axB), (axC, axD)) = plt.subplots(
nrows=2, ncols=2, figsize=(15.0, 18.0)
)
P = Pvar
T = Tref
c = cref
Tm = np.array(
[par_i.Tm0 + P/par_i.clap for par_i in par]
).transpose()
axA.plot(
P, Tm[:, 0] - 273., '-k',
linewidth=2, label=r'$T^\mathcal{S}_1$'
)
axA.plot(
P, Tm[:, 1] - 273., '--k',
linewidth=2, label=r'$T^\mathcal{S}_2$'
)
axA.set_xlim(0.0, 7.0)
axA.set_xlabel('$P$, GPa', fontsize=20)
axA.set_ylim(900, 2000)
axA.set_ylabel(r'$T$, $^\circ$C', fontsize=20)
axA.text(
0.01, 1990, r'(a)', fontsize=20,
verticalalignment='top', horizontalalignment='left'
)
axA.tick_params(axis='both', which='major', labelsize=15)
axA.legend(loc='lower right', fontsize=15)
P = Pref
T = Tvar
c = cref
K = np.array(
[
[
ParCoef_IdealSolution(T_i, P, par_j) for par_j in par
]
for T_i in T
]
)
axB.plot(
T - 273., K[:, 0], '-k',
linewidth=2, label=r'$\check{K}_1$'
)
axB.plot(
T - 273., K[:, 1], '--k',
linewidth=2, label=r'$\check{K}_2$'
)
axB.set_yscale('log')
axB.set_xlim(1050, 1950)
axB.set_xticks((1200., 1400., 1600., 1800.))
axB.set_xticklabels((1200, 1400, 1600, 1800))
axB.set_xlabel(r'$T$, $^\circ$C', fontsize=20)
axB.set_ylim(0.01, 100)
axB.set_ylabel(r'${c}^s_j/{c}^\ell_j$', fontsize=20)
axB.text(
1050, 95.0, r'(b)', fontsize=20,
verticalalignment='top', horizontalalignment='left'
)
axB.tick_params(axis='both', which='major', labelsize=15)
axB.legend(fontsize=15)
T = Tvar
P = Pref
c = cref
Theta = EquilibriumState(
len(par), c, T, P, par, ParCoef_IdealSolution
)
axD.plot(
Tvar - 273.,
[t.f for t in Theta],'-k','linewidth',2
)
axD.set_xlim(1200., 1750.)
axD.set_xticks(
(1200., 1300., 1400., 1500., 1600., 1700.)
)
axD.set_xticklabels(
(1200, 1300, 1400, 1500, 1600, 1700)
)
axD.set_ylim(-0.02, 1.02)
axD.set_xlabel(r'$T$, $^\circ$C', fontsize=20)
axD.set_ylabel(r'$\check{f}$, wt. frac.', fontsize=20)
axD.tick_params(axis='both', which='major', labelsize=15)
axD.text(
1200.0, 1.01, r'(d)', fontsize=20,
verticalalignment='top', horizontalalignment='left'
)
cl_hold = np.array([t.cl[1] for t in Theta])
cs_hold = np.array([t.cs[1] for t in Theta])
f_hold = np.array([t.f for t in Theta])
cl_hold[f_hold < 1e-6] = np.nan
cs_hold[1.-f_hold < 1e-6] = np.nan
c = cvar
P = Pref
T = Tref
Theta2 = EquilibriumState(
len(par), c, T, P, par, ParCoef_IdealSolution
)
axC.plot(
c[:, 1], [t.Ts - 273. for t in Theta2], '-',
linewidth=4, color=[0.7, 0.7, 0.7]
)
axC.plot(
c[:, 1], [t.Tl - 273. for t in Theta2], '-',
label='phase boundary', linewidth=4, color=[0.7, 0.7, 0.7]
)
axC.plot(
cl_hold, Tvar - 273., '--k',
label='$\check{c}^\ell_2$', linewidth=2
)
axC.plot(
cs_hold, Tvar - 273., '-k',
linewidth=2, label='$\check{c}^\ell_2$'
)
axC.set_xlim(0.0, 1.0)
axC.set_xlabel('${c}^i_2$, wt. frac.', fontsize=20)
axC.set_ylabel('$T$, $^\circ$C', fontsize=20)
axC.text(
0.005, 1990, '$(c)$', fontsize=20,
verticalalignment='top', horizontalalignment='left'
)
axC.text(0.22, 1400, 'solid$+$liquid', fontsize=20)
axC.text(0.72, 1500,'liquid', fontsize=20)
axC.text(0.05, 1150,'solid', fontsize=20)
axC.tick_params(axis='both', which='major', labelsize=15)
axC.legend(fontsize=15)
fig.supxlabel("Figure 10.1", fontsize=20)
plt.show()
Application to a three-pseudo-component system¶
par3 = [
IdealSolutionParameters(600e3, 70., 1./50., 1780. + 273.),
IdealSolutionParameters(450e3, 30., 1./100., 950. + 273.),
IdealSolutionParameters(330e3, 30., 1./50., 410. + 273.)
]
Pref = 1.
Pvar = np.linspace(0.0, 7.0, 20)
Tref = 1350. + 273.
Tvar = np.linspace(1000., 1700. , 200) + 273.
cref = np.array([0.75, 0.248, 0.002])
npts = 100
conc_water_in_pure_component = 1e-4/cref[2]
Figures 10.2 below plot the ideal-solution model of a three-component system of “olivine” (\(j=1\)), “basalt” (\(j=2\)) and “hydrous basalt” (\(j=3\)). Parameter values are given in table above. (a) Pure-component melting temperatures \(\melttemp_j\) as a linear function of pressure. (b) Partition coefficients \(\parco_j\) as a function of temperature at a constant pressure of 1 GPa. (d) Equilibrium melt fraction as a function of temperature for a bulk composition of 75 wt% olivine component with and without 0.2 wt% of hydrated basalt component at 1 GPa.
fig, (axA, axB, axD) = plt.subplots(
nrows=3, ncols=1, figsize=(6., 27.)
)
P = Pvar
T = Tref
c = cref
Tm = np.array(
[par_i.Tm0 + P/par_i.clap for par_i in par3]
).transpose()
axA.plot(
P, Tm[:, 0] - 273., '-k',
linewidth=2, label=r'$T^\mathcal{S}_1$'
)
axA.plot(
P, Tm[:, 1] - 273., '--k',
linewidth=2, label=r'$T^\mathcal{S}_2$'
)
axA.plot(
P, Tm[:, 2] - 273., '-.k',
linewidth=2, label=r'$T^\mathcal{S}_3$'
)
axA.set_xlim(0.0, 7.0)
axA.set_xlabel('$P$, GPa', fontsize=20)
axA.set_ylim(100., 2100.)
axA.set_ylabel(r'$T$, $^\circ$C', fontsize=20)
axA.set_yticks((500, 1000, 1500, 2000))
axA.text(
0.01, 2080., r'(a)', fontsize=20,
verticalalignment='top', horizontalalignment='left'
)
axA.tick_params(axis='both', which='major', labelsize=13)
axA.legend(loc='lower right', fontsize=15, ncol=3)
P = Pref
T = Tvar
c = cref
K = np.array(
[
[
ParCoef_IdealSolution(T_i, P, par_j)
for par_j in par3
]
for T_i in T
]
)
axB.plot(
T - 273., K[:, 0], '-k',
linewidth=2, label=r'$\check{K}_1$'
)
axB.plot(
T - 273., K[:, 1], '--k',
linewidth=2, label=r'$\check{K}_2$'
)
axB.plot(
T - 273., K[:, 2], '-.k',
linewidth=2, label=r'$\check{K}_3$'
)
axB.set_yscale('log')
axB.set_xlim(1000., 1700.)
axB.set_xlabel(r'$T$, $^\circ$C', fontsize=20)
axB.set_ylim(1e-5, 100.)
axB.set_ylabel(r'${c}^s_j/{c}^\ell_j$', fontsize=20)
axB.set_yticks((1e-4, 1e-2, 1e0))
axB.text(
1005., 90., r'(b)', fontsize=20,
verticalalignment='top', horizontalalignment='left'
)
axB.tick_params(axis='both', which='major', labelsize=13)
axB.legend(fontsize=15)
T = Tvar
P = Pref
cwet = cref
Theta_wet = EquilibriumState(
len(par3), cwet, T, P, par3, ParCoef_IdealSolution
)
cdry = np.array([0.75, 0.25, 0.])
Theta_dry = EquilibriumState(
len(par3), cdry, T, P, par3, ParCoef_IdealSolution
)
axD.plot(
Tvar - 273., [t_wet.f for t_wet in Theta_wet],
'-k', linewidth=2, label='wet'
)
axD.plot(
Tvar - 273., [t_dry.f for t_dry in Theta_dry],
'--k', linewidth=2, label='dry'
)
axD.set_xlabel('$T$, $^\circ$C', fontsize=20)
axD.set_ylabel('$\check{f}$, wt. frac.', fontsize=20)
axD.set_xlim(1000., 1500.)
axD.set_ylim(0., 0.4)
axD.set_yticks((0., 0.1, 0.2, 0.3, 0.4))
axD.text(
1000., 0.396,'(d)', fontsize=20,
verticalalignment='top', horizontalalignment='left'
)
axD.tick_params(axis='both', which='major', labelsize=13)
axD.legend(fontsize=15, loc='lower right')
cl_hold = np.array([th.cl for th in Theta_wet])
cs_hold = np.array([th.cs for th in Theta_wet])
f_hold = np.array([th.f for th in Theta_wet])
cl_hold[f_hold<1e-6] = np.nan
cs_hold[1-f_hold<1e-6] = np.nan
fig.supxlabel("Figure 10.2", fontsize=20)
plt.show()
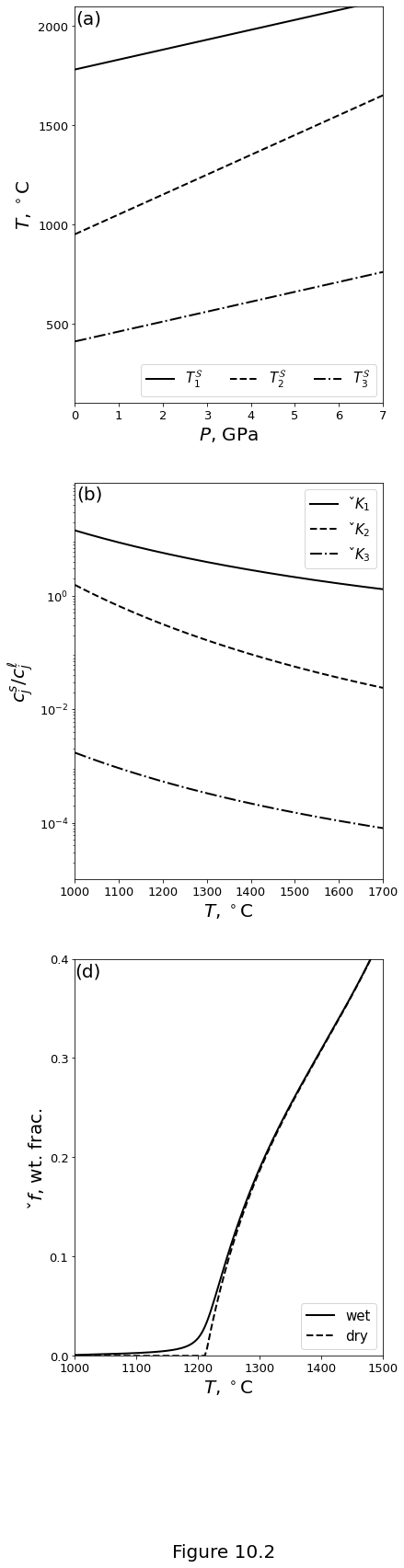
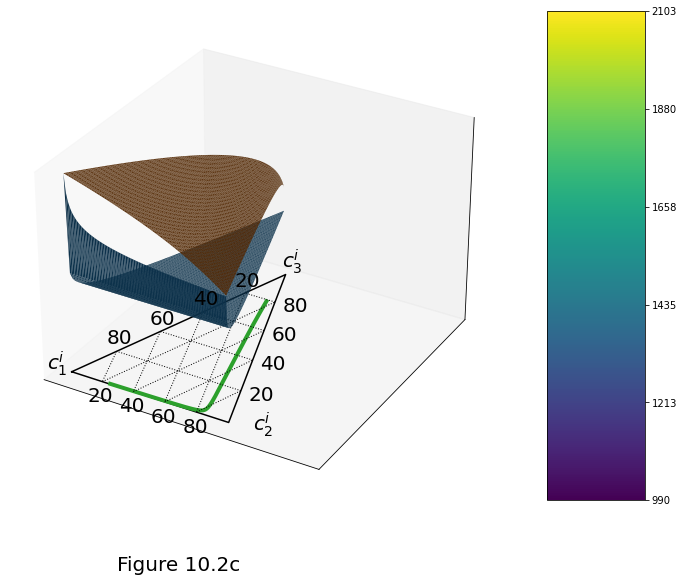
Figure 10.2c below plot the ideal-solution model of a three-component system of “olivine” (\(j=1\)), “basalt” (\(j=2\)) and “hydrous basalt” (\(j=3\)). Parameter values are given in table above. (c) Surfaces are the solidus and liquidus temperature through the full, 3-component space at a pressure of 1 GPa. The composition along the solidus surface refers to the solid phase; that along the liquidus surface refers to the liquid phase. The compositional evolution of the melt for a closed system with 75 wt% olivine and 0.2 wt% hydrous basalt is shown by the solid line. It starts at the triangle and progresses toward the circle with increasing \(\temp\) and \(\equi\mf\).
C3 = np.linspace(0., 1., npts)
C2 = np.linspace(0., 1., npts)
[C2,C3] = np.meshgrid(C2,C3)
C2 = np.reshape(C2, npts*npts)
C3 = np.reshape(C3, npts*npts)
C1 = 1-C2-C3
C = np.zeros((npts*npts, 3), dtype=float)
C[:, 0] = C1
C[:, 1] = C2
C[:, 2] = C3
P = Pref
T = Tref
Theta = EquilibriumState(
len(par3), C, T, P, par3, ParCoef_IdealSolution
);
Tsol = np.array([th.Ts for th in Theta])
Tliq = np.array([th.Tl for th in Theta])
Tsol[C[:,1]+C[:,2]>=1] = np.nan
Tliq[C[:,1]+C[:,2]>=1] = np.nan
def terncoords(c1,c2,c3):
csum = c1 + c2 + c3
c1 = c1/csum
c2 = c2/csum
c3 = c3/csum
y = c2 * np.sin(np.pi/3.)
x = c1 + y / np.tan(np.pi/3.)
return x, y
X, Y = terncoords(C2, C3, C1)
triangXY = mtri.Triangulation(X, Y)
fig, axC = plt.subplots(
figsize=(18., 9.),
subplot_kw={"projection": "3d"}
)
axC.plot_trisurf(triangXY, Tsol)
surf = axC.plot_trisurf(triangXY, Tliq)
axC.plot(
[0., 1., 0.5, 0.],
[0., 0., np.sin(np.pi/3.), 0.], color=[0,0,0]
)
x, y = terncoords(cl_hold[:,1], cl_hold[:,2], cl_hold[:,0])
axC.plot(x, y, zs=0, zdir='z', linewidth=4)
m = 5
grids = np.linspace(0, 100, m+1)
grids = grids[:-1]
x3, y3 = terncoords(100-grids, grids, np.zeros_like(grids))
x2, y2 = terncoords(grids, np.zeros_like(grids), 100-grids)
x1, y1 = terncoords(np.zeros_like(grids), 100-grids, grids)
n = m-1
for i in np.arange(1, m):
axC.plot(
[x1[i], x2[n-i+1]], [y1[i], y2[n-i+1]],
'k:', zs=0, zdir='z', linewidth=1
)
axC.plot(
[x2[i], x3[n-i+1]], [y2[i], y3[n-i+1]],
'k:', zs=0, zdir='z', linewidth=1
)
axC.plot(
[x3[i], x1[n-i+1]], [y3[i], y1[n-i+1]],
'k:', zs=0, zdir='z', linewidth=1
)
axC.text(0.44, 0.9, z=0., s='${c}_3^i$', fontsize=20)
axC.text(1.15, 0.0, z=0., s='${c}_2^i$', fontsize=20)
axC.text(-0.13,-0.030, z=0., s='${c}_1^i$', fontsize=20)
labels = [str(int(g)) for g in grids[1:]]
for x3_, y3_, l_ in zip(x3[1:], y3[1:], labels):
axC.text(x3_ + 0.09, y3_ - 0.04, z=0., s=l_, fontsize=20)
for x2_, y2_, l_ in zip(x2[1:], y2[1:], labels):
axC.text(x2_ + 0.04, y2_ - 0.12, z=0., s=l_, fontsize=20)
for x1_, y1_, l_ in zip(x1[1:], y1[1:], labels):
axC.text(x1_ - 0.09, y1_ + 0.02, z=0., s=l_, fontsize=20)
axC.set_xticks(())
axC.set_yticks(())
axC.set_zticks(())
fig.supxlabel("Figure 10.2c", fontsize=20)
ticks_ = (0., 0.2, 0.4, 0.6, 0.8, 1.)
cbar = fig.colorbar(surf, aspect=5, ticks=(ticks_))
cticks_ = (
int(
(np.nanmax(Tliq) - np.nanmin(Tliq))*s
+ np.nanmin(Tliq)
) for s in ticks_
)
cbar.ax.set_yticklabels((cticks_));
Approaching the eutectic phase diagram¶
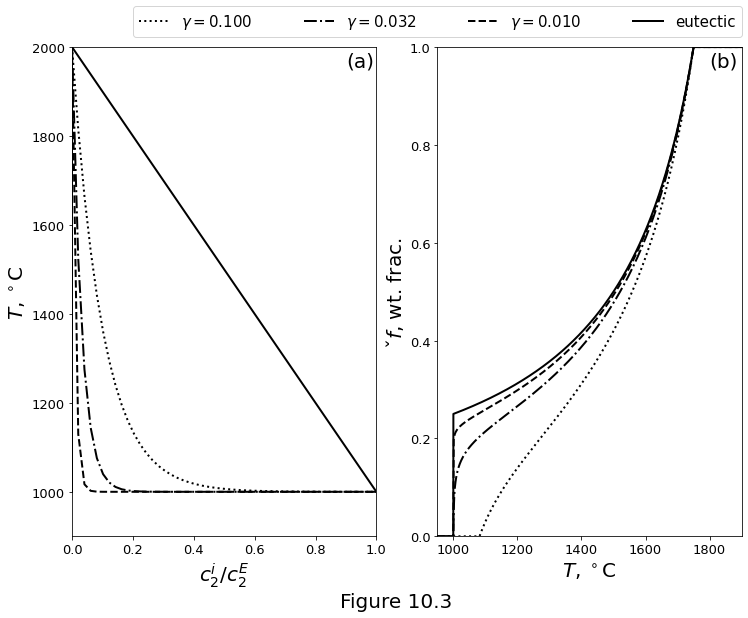
Figure 10.3 plots a eutectic phase diagram compared with ideal-solution phase loops for different values of \(R_2\). Other ideal-solution parameters are \(R_1=60\) J/kg/K, \(\latent_1=\latent_2=500\) kJ/kg. (a) Solidus and liquidus curves computed with ideal solution theory compared with the eutectic solidus and liquidus. (b) Isobaric melting curves computed based on the phase loops and the eutectic.
def liquidusQuadratic(H, C, L, cp, T0, Te):
a = L
b = cp*(T0-Te)-H
c = -C*cp*(T0-Te)
return (-b + np.sqrt(b*b - 4*a*c))/2/a
def equilibriumEutectic(H, C, T0, Te, L, cp):
He_low = 0
He_high = L*C[1]
f = np.array([
0 if Hj <= He_low else
Hj/L if Hj <= He_high else
np.minimum(
liquidusQuadratic(
Hj, C[1], L, cp, T0, Te
),
1
) for Hj in H
])
T = Te + (H - L * f)/cp
return T, f
def pseudoEutecticSolidus(C, T0, Te, gamma):
return T0 + (Te - T0) * (
1. - np.exp(-C/gamma)
)/(
1.-np.exp(-1/gamma)
)
def pseudoEutecticLiquidus(C, T0, Te, gamma):
return T0 + (Te - T0) * C
def equilibriumPseudoEutectic(T, C, T0, Te, L, gamma):
pES = pseudoEutecticSolidus(C, T0, Te, gamma)
pEL = pseudoEutecticLiquidus(C, T0, Te, gamma)
cl = np.array([
np.nan if Tj < pES else
C if Tj > pEL else
(Tj-T0)/(Te-T0) for Tj in T
])
cs = np.array([
C if Tj < pES else
np.nan if Tj > pEL else
-gamma*np.log(
1-cl_j*(1-np.exp(-1/gamma))
) for Tj, cl_j in zip(T, cl)
])
f = np.array([
0.0 if Tj < pES else
1.0 if Tj > pEL else
(
C - cs_j
)/(
cl_j - cs_j
) for Tj, cl_j, cs_j in zip(T, cl, cs)
])
return f
fig, ((axA, axB)) = plt.subplots(
nrows=1, ncols=2, figsize=(12., 9.)
)
npts = 500
Tref = 1350
Tvar = np.linspace(950, 1900, npts)
cref = np.array([0.75, 0.25])
Pref = 1
P = np.linspace(0.0, 7.0, 300)
T0 = 2000
Te = 1000
L = 500e3
gamma_arr = np.flip(np.logspace(-2, -1, 3))
plotstyle = [':k', '-.k', '--k']
cp = 1200
H = np.linspace(-L, L + cp*(T0-Te), 1000)
T = np.linspace(T0, Te)
X = (T-Te)/(T0-Te)
C = 1 - X
axA.plot(C, T,'-k', linewidth=2)
axA.plot([0, 0], [Te, T0],'-k', linewidth=2)
for gamma, pltstyle in zip(gamma_arr, plotstyle):
Ts = pseudoEutecticSolidus(C, T0, Te, gamma)
axA.plot(C, Ts, pltstyle, linewidth=2)
axA.set_ylabel('$T$, $^\circ$C', fontsize=20)
axA.set_xlim((0, 1))
axA.set_xlabel('${c}^i_2/c^E_2$', fontsize=20)
axA.set_ylim((900, 2000))
axA.text(
0.9, 1990.,'(a)', fontsize=20,
verticalalignment='top', horizontalalignment='left'
)
axA.tick_params(axis='both', which='major', labelsize=13)
TE, fE = equilibriumEutectic(H, cref, T0, Te, L, cp)
T = np.sort(np.hstack((Tvar, Te)))
plots = []
labels = []
for gamma, pltstyle in zip(gamma_arr, plotstyle):
theta_f = equilibriumPseudoEutectic(
T, cref[1], T0, Te, L, gamma
)
plots.append(
axB.plot(
T, theta_f, pltstyle, linewidth=2
)
)
labels.append('$\gamma=${:.3f}'.format(gamma))
plots.append(axB.plot(TE, fE, '-k', linewidth=2))
labels.append('eutectic')
axB.set_xlim([950, 1900])
axB.set_ylim([0, 1])
axB.set_xlabel('$T$, $^\circ$C', fontsize=20)
axB.set_ylabel('$\check{f}$, wt. frac.', fontsize=20)
axB.text(
1800, 0.99, '(b)', fontsize=20,
verticalalignment='top', horizontalalignment='left'
)
axB.tick_params(axis='both', which='major', labelsize=13)
handles = [p[0] for p in plots]
plt.legend(
handles=handles, fontsize=15, labels=labels,
bbox_to_anchor=(-1.0, 1.02, 2., .2), loc='lower left',
ncol=4, mode="expand", borderaxespad=0.
)
fig.supxlabel("Figure 10.3", fontsize=20)
plt.show()
Linearising the two-component phase diagram¶
Phase diagrams constructed with ideal solution theory provide a useful basis for developing the melting relations required for magma/mantle dynamics. Their nonlinearity, however, precludes a use in analytical calculations; instead they must be handled numerically. It is therefore important to formulate an approximation in which the equilibrium state is computed analytically.
For linear variations in pressure and composition, we can write
where \(\solslope\) is the constant slope of the solidus with concentration in the two component space and \(\soltemp_\text{ref}\) is a reference temperature at \(\pres=\pres_\text{ref}\) and \(\equi\con\sol=\equi\con\sol_\text{ref}\). Also, \(\Delta\equi\con \equiv \equi\con\sol - \equi\con\liq\) is the concentration difference between the solidus and the liquidus. If \(\Delta\equi\con\) is taken to be a constant then the liquidus slope \(\liqslope\) and the solidus slope \(\solslope\) are equal.
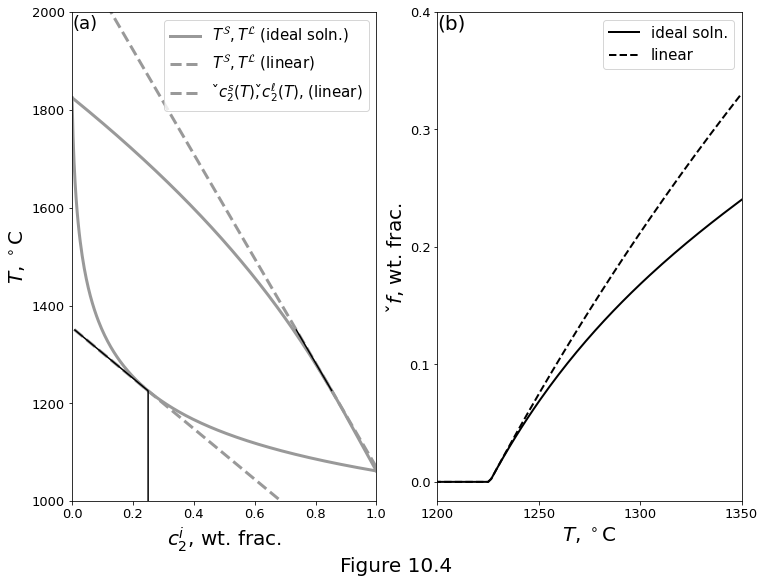
Figure 10.4 below plots a comparison of a two-component, ideal-solution phase loop with a linearised solidus and liquidus at 1 GPa. The phase loop uses the parameters from the Table above. (a) Solidus and liquidus curves. Evolution of an initially unmolten rock with 25 wt% basaltic component for increasing temperature, along the linearised phase boundaries. (b) Isobaric melting curves.
def SolLiqTemperature(C, Cref, M, Tref):
return Tref + M*(C-Cref)
def SolLiqComposition(T, Tref, M, Cref):
return Cref + (T - Tref)/M
def MeltFraction(T, C, ML, MS, LCref, SCref, Tref):
TS = SolLiqTemperature(C, SCref, MS, Tref)
TL = SolLiqTemperature(C, LCref, ML, Tref)
Cs = SolLiqComposition(T, Tref, MS, SCref)
Cl = SolLiqComposition(T, Tref, ML, LCref)
F = (C - Cs)/(Cl - Cs)
F[T <= TS] = 0.
F[T >= TL] = 1.
Cs[T < TS] = C
Cl[T < TS] = np.nan
return F, Cs, Cl
par_lin = [
IdealSolutionParameters(600e3, 70., 1./45., 1780. + 273.),
IdealSolutionParameters(450e3, 30., 1./112., 950. + 273.)
]
Pref = 1.
Pvar = np.linspace(0., 7., 20)
Tref = 1350. + 273.
Tvar = np.linspace(900., 1350., 300) + 273.
cref = np.array([0.75, 0.25])
cvar = np.zeros((300, 2), dtype=float)
cvar[:, 0] = np.linspace(0., 1., 300)
cvar[:, 1] = 1.0 - np.linspace(0., 1., 300)
fig, ((axA, axB)) = plt.subplots(
nrows=1, ncols=2, figsize=(12.0, 9.0)
)
c = cvar
T = Tref
P = Pref
Theta = EquilibriumState(
len(par_lin), c, T, P, par_lin, ParCoef_IdealSolution
)
Cint = cref[1]*np.array([0.999, 1.001])
f_Tint = interp1d(
c[:,1], np.array([th.Ts for th in Theta]) - 273.
)
Tint = f_Tint(Cint)
MS = (Tint[1]-Tint[0])/(Cint[1]-Cint[0])
Tref = np.mean(Tint)
f_Cint = interp1d(
np.array([th.Tl for th in Theta]) - 273, c[:,1]
)
Cint = f_Cint(Tint)
ML = (Tint[1]-Tint[0])/(Cint[1]-Cint[0])
Cref = np.mean(Cint)
F, Cs, Cl = MeltFraction(
Tvar - 273., cref[1], ML, MS, Cref, cref[1], Tref
)
plots = []
axA.plot(
c[:,1], np.array([th.Ts for th in Theta]) - 273.,
'-', linewidth=3, color=[0.6, 0.6, 0.6]
)
plots.append(
axA.plot(
c[:,1], np.array([th.Tl for th in Theta]) - 273., '-',
linewidth=3, color=[0.6, 0.6, 0.6]
)
)
plots.append(
axA.plot(
c[:,1], SolLiqTemperature(c[:,1], Cref, ML, Tref),
'--', color=[0.6, 0.6, 0.6], linewidth=3
)
)
plots.append(
axA.plot(
c[:,1], SolLiqTemperature(c[:,1], cref[1], MS, Tref),
'--', color=[0.6, 0.6, 0.6], linewidth=3
)
)
axA.plot(Cs, Tvar-273, '-k', linewidth=1.5)
axA.plot(Cl, Tvar-273, '-k', linewidth=1.5)
axA.set_ylabel('$T$, $^\circ$C', fontsize=20)
axA.set_xlabel('${c}^i_2$, wt. frac.', fontsize=20)
axA.set_xlim([0, 1])
axA.set_ylim([1000, 2000])
axA.tick_params(axis='both', which='major', labelsize=13)
axA.text(0.001, 1965., '(a)', fontsize=18)
labels=[
'$T^\mathcal{S}, T^\mathcal{L}$ (ideal soln.)',
'$T^\mathcal{S},T^\mathcal{L}$ (linear)',
'$\check{c}^s_2(T),\check{c}^\ell_2(T)$, (linear)'
]
axA.legend(
handles=([p[0] for p in plots]),
fontsize=15, labels=labels
)
T = Tvar
P = Pref
c = cref
Theta = EquilibriumState(
len(par_lin), c, T, P, par_lin, ParCoef_IdealSolution
)
axB.plot(
T - 273., np.array([th.f for th in Theta]),
'-k', linewidth=2, label='ideal soln.'
)
axB.plot(T - 273., F,'--k', linewidth=2, label='linear')
axB.set_xlabel('$T$, $^\circ$C', fontsize=20)
axB.set_xlim([1200, 1350])
axB.set_xticks((1200, 1250, 1300, 1350))
axB.set_ylabel('$\check{f}$, wt. frac.', fontsize=20)
axB.set_yticks((0., 0.1, 0.2, 0.3, 0.4))
axB.text(1200.01, 0.385, '(b)', fontsize=20)
axB.tick_params(axis='both', which='major', labelsize=13)
axB.legend(fontsize=15)
fig.supxlabel("Figure 10.4", fontsize=20)
plt.show()